Qualification of pharmaceutical plants
(German article)
Practical aspects of reporting, master plan and risk analysis
For manufacturers of pharmaceutical products, the performance of equipment qualification is an elementary component of the quality management system and a prerequisite for obtaining and maintaining manufacturing authorization.
According to the definition of the EU GMP regulations, the manufacturer of pharmaceutical products proves with the qualification of his equipment that it is suitable for the intended purpose. (Article in German language)
- authors: Jens Lehmann, Wolfgang Röper, Glatt Ingenieurtechnik GmbH
- originally published in the magazine ‘CHEManager’, issue 3-4 2012, Wiley-VCH Verlag GmbH & Co. KGaA


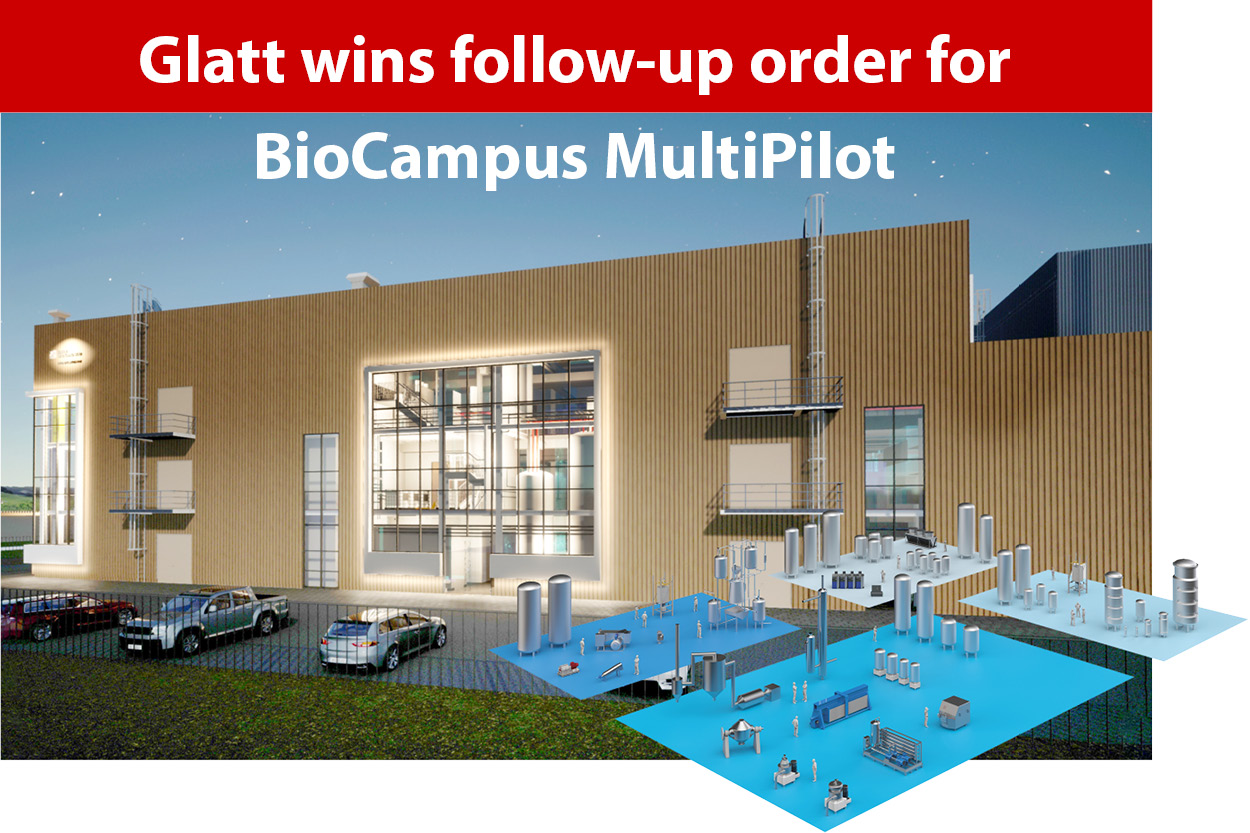



 Copyright: publish-industry Verlag GmbH
Copyright: publish-industry Verlag GmbH Copyright: Konradin-Verlag Robert Kohlhammer GmbH
Copyright: Konradin-Verlag Robert Kohlhammer GmbH