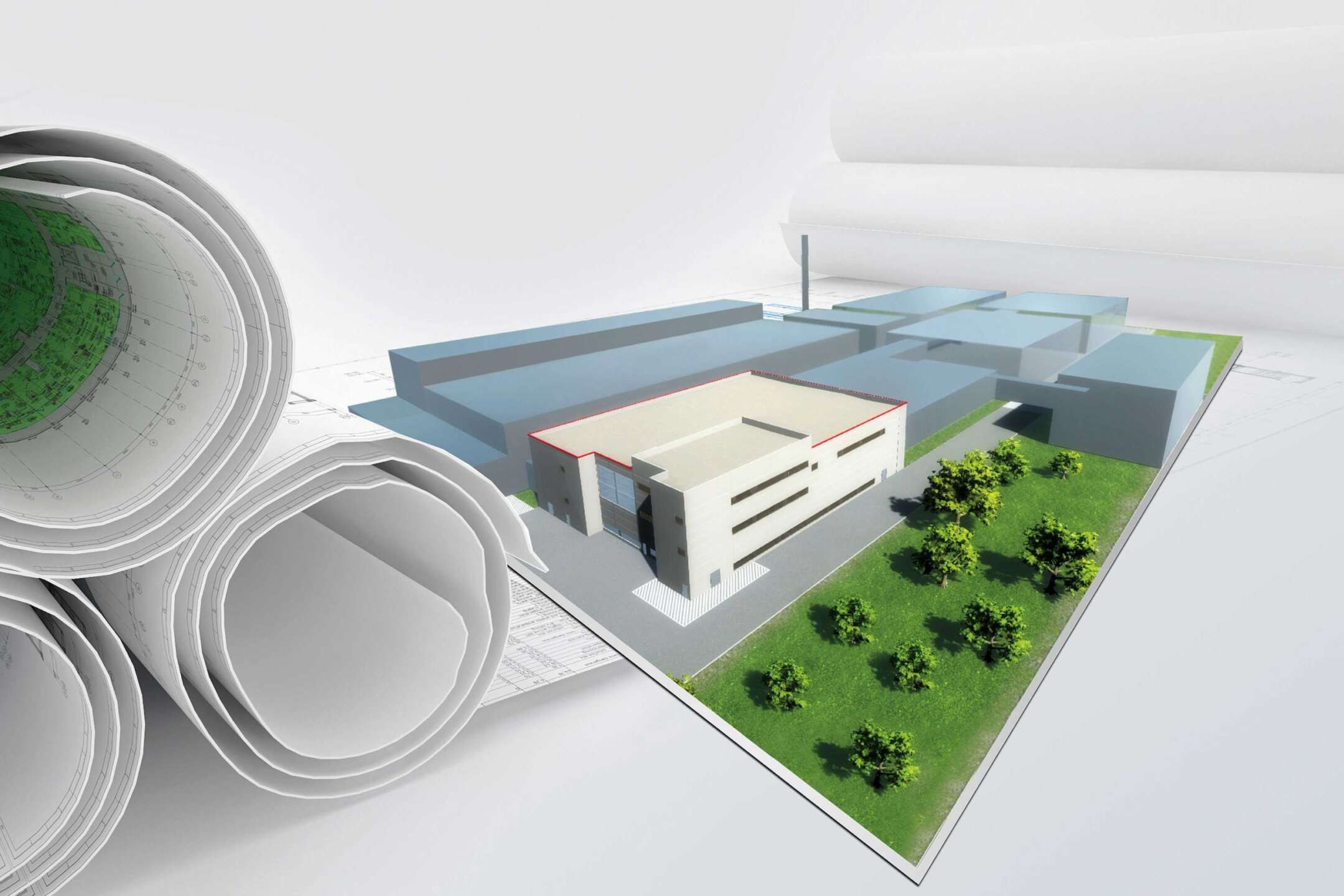
Planned fill & finish plant for vaccines in Ghana, east side view
Glatt to inaugurate vaccine production in Ghana with fill & finish plant
February 2022: Glatt Ingenieurtechnik in Weimar, Germany, has been commissioned to conduct a concept study to investigate the technical and economic aspects of a fill & finish plant for vaccines. The independent, fully integrated state-of-the-art biotechnological facility is to be built in Accra, Ghana, and will lay the foundation for local vaccine production against COVID-19 and other diseases.
The facility will serve as a blueprint to establish local vaccine production and is part of the government’s Vaccine Development and Manufacturing Presidential Committee (VMC) strategy. Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) has commissioned Glatt Ingenieurtechnik for phase 1 of the project.
The current pandemic has underscored the need for local vaccine production in Africa. Through the VMC, Ghanaian President Nana Addo Dankwa Akufo-Addo has convened a board to establish a 10-year plan for vaccine development and production. The most promising near-term approach to achieve this goal is the construction of a bottling and packaging (fill & finish) plant. On this basis, further capacity for full vaccine production in Africa is to be developed.
As a process expert, and working in close collaboration with the vaccine manufacturer, Glatt will provide detailed planning services for the plant, investigate its technical feasibility and provide economic efficiency advice. The current planning envisages the filling of vaccines in four independent production modules. Single-use components for different primary packaging materials will be used. The plant will be operated by DEK Vaccines, a company founded by three local pharmaceutical manufacturers.
Step by step to complete vaccine production
The project comprises three phases, which will be implemented on a step-by-step basis. The specific requirements for the manufacture of current and future products are already being incorporated into Glatt’s planning to ensure a smooth production process later on. The same approach is being applied to legal conditions and standards.
The main building and administration wing will occupy 7000 square meters, encompassing various essential areas on different floors; these include the warehouse and logistics level, a mezzanine for changing rooms with an adjacent canteen and a floor for administration and technology. The heart of the facility will comprise the production level, two preparation and formulation units for different vaccine categories (up to biosafety level 1), the R&D area and a quality control laboratory.
With successful completion of all phases, the plant will be able to fill at least 100 million vaccine doses per shift per year.





 Copyright: Vogel Communications Group
Copyright: Vogel Communications Group Copyright: ZVH Straubing
Copyright: ZVH Straubing